Pipeline
We are advancing a proprietary portfolio of TCR-T product candidates, for which we retain worldwide commercial rights.
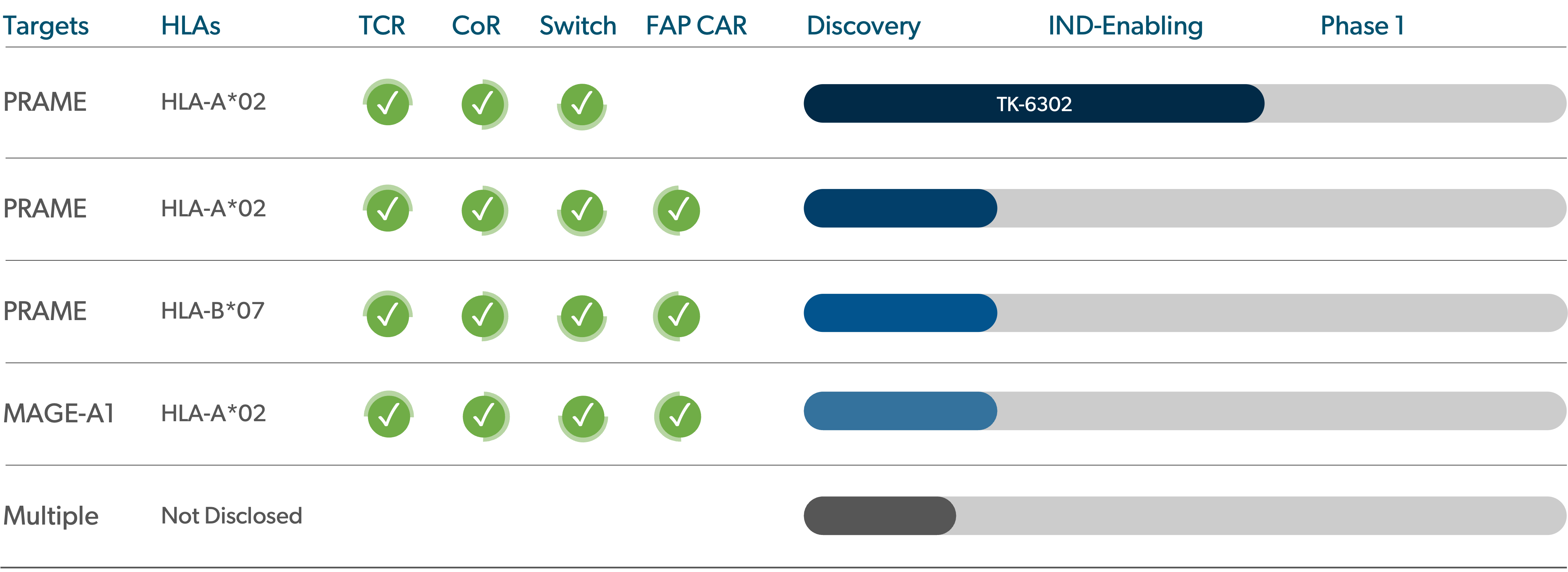
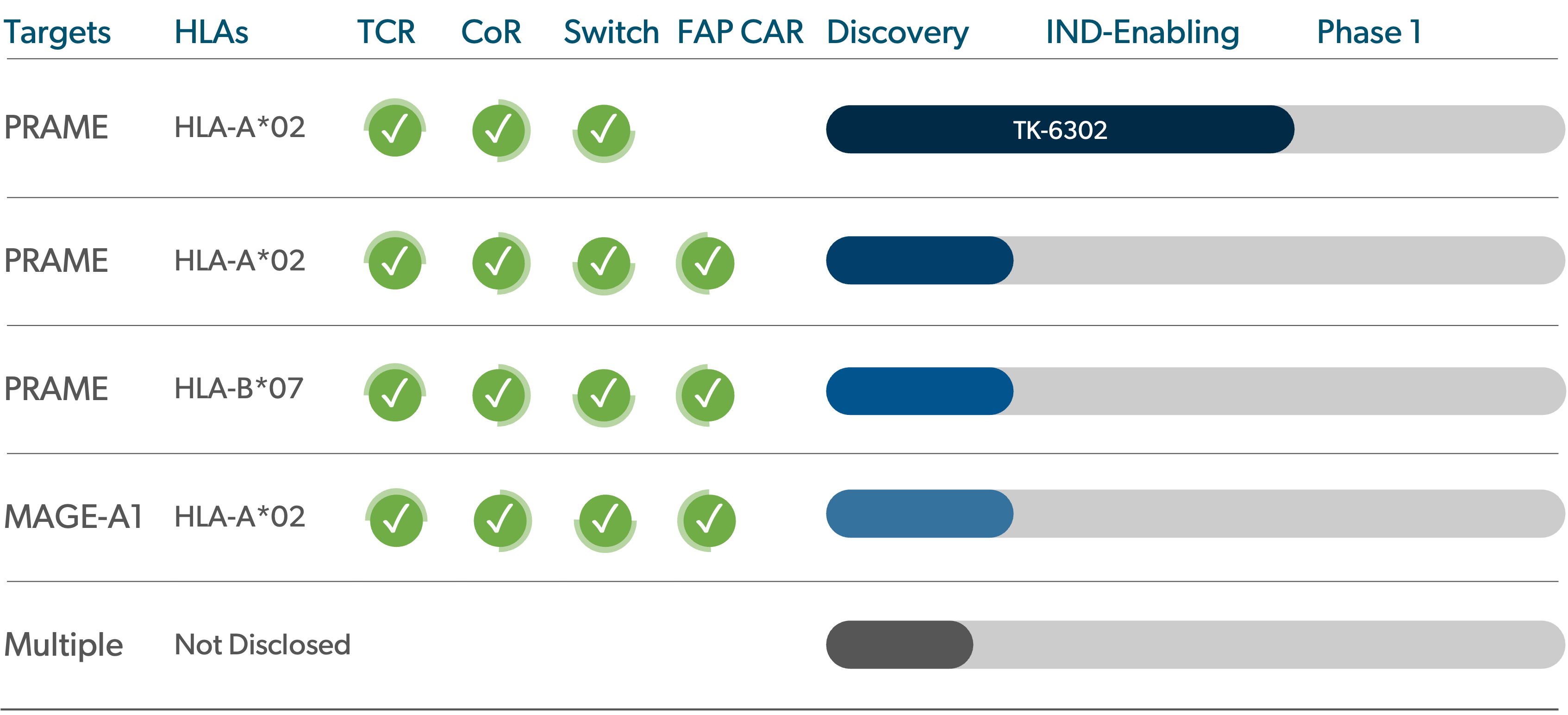
PRAME is found in cancers of the skin, uterus, ovaries, lungs, breasts, esophagus, kidneys, cervix, and head & neck.
MAGE-A1 is overexpressed in cancers of the colon, skin, brain, lungs, prostate, and breasts.
PRAME-targeting TCR-T: TK-6302
TK-6302 is T-knife’s lead TCR-T and targets PRAME, an antigen expressed in a number of solid tumors. TK-6302 T cells are supercharged, as they have been engineered to include T-knife’s proprietary platform of technologies to improve T cell fitness and persistence within an immunosuppressive tumor microenvironment, and to improve durability of response. In preclinical studies, TK-6302 has demonstrated best-in-class preclinical anti-tumor efficacy as compared to other PRAME TCR-T approaches, providing the potential for broad, deep and durable responses in solid tumor indications.
TK-6302 destroys multiple rounds of tumors in a complex 3-dimensional (3D) spheroid tumor model
Note: 3D spheroid tumor models are engineered to mimic the complex microenvironment T cells face in the tumor, including diverse inhibitory molecules and a variety of cells such as stromal fibroblasts and monocytes.

