Cell Therapy
Cell therapy is the use of human cells as a treatment for disease. T cell therapy has become a powerful modality to treat cancers. There are several FDA approved T-cell-based therapies for the treatment of several cancers. However, there remains a significant need to develop cell therapies that are effective against other cancer types, especially for difficult-to-treat solid tumors.
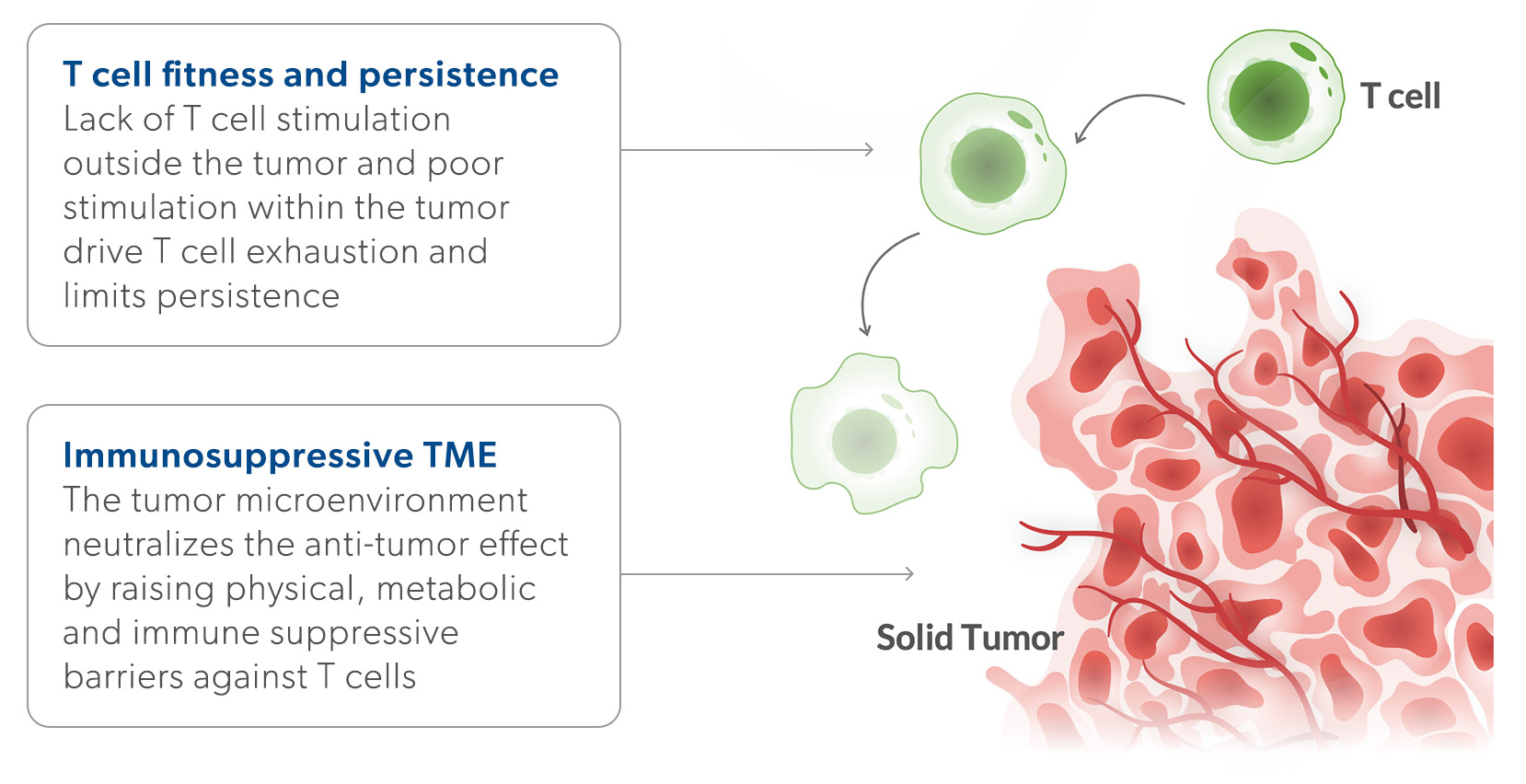
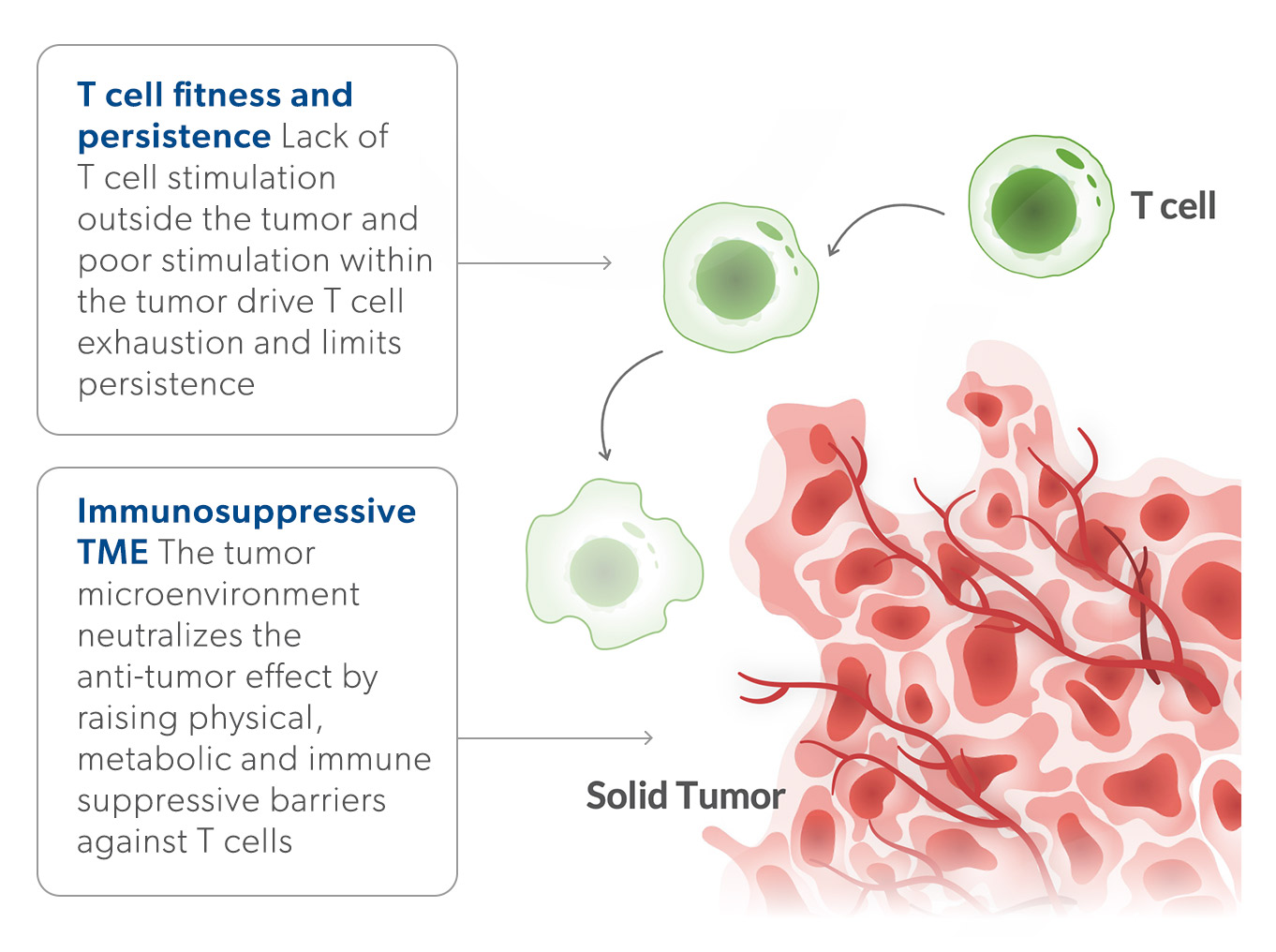
The Challenge: Response reates and durability have been limited by poor T cell fitness and a hostile tumor microenvironment
Our Approach: Supercharged TCR Therapies (TCR-Ts)
T cells play a key role in the immune response by directly recognizing and eliminating infected, foreign or altered cells, such as cancer cells. To do this, they use their T-cell receptors (TCRs) to scan the surface of other cells for foreign antigens presented on Human Leukocyte Antigen (HLA) complexes.
Cancer cells can be recognized by mutated or viral antigens expressed only in the tumor, or self-antigens normally expressed during embryonic development and in non-somatic adult tissues. Genetic engineering of T cells with TCRs recognizing antigens aberrantly or over-expressed in cancers can redirect these T cells to the tumor, potentially offering curative responses to cancer patients.
TCR-Ts are a promising therapeutic modality for solid tumors.
- Harness the natural activation mechanism of T cells
- Stimulate a powerful immune response
- Result in direct cancer cell death and cytotoxicity on the tumor microenvironment
- Target 100% of the cancer proteome, both cell surface & intracellular targets
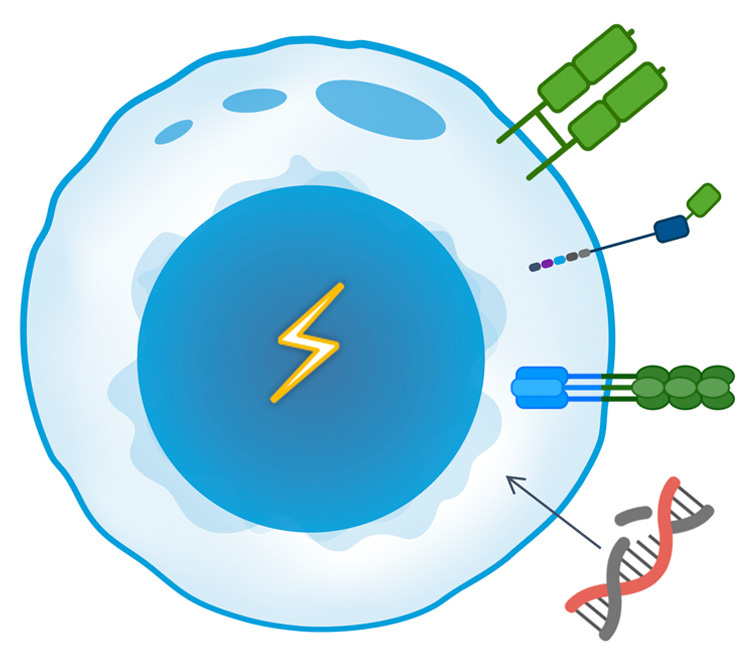
T-knife is developing supercharged TCR-Ts that incorporate multiple best-in-class technologies and are rationally designed to target specific tumor types. T-knife’s TCR-Ts are “supercharged” by engineering the cell therapy with multiple enhancements designed to improve response rates and durability with the goal of delivering transformative outcomes in the treatment of solid tumor cancers.
High Affinity TCRs
- Proprietary MyT platform, a high-throughput technology for generating TCRs with optimized affinity / specificity profiles
- Avoids immune central tolerance mechanisms; higher affinity relative to TCRs isolated from human donors
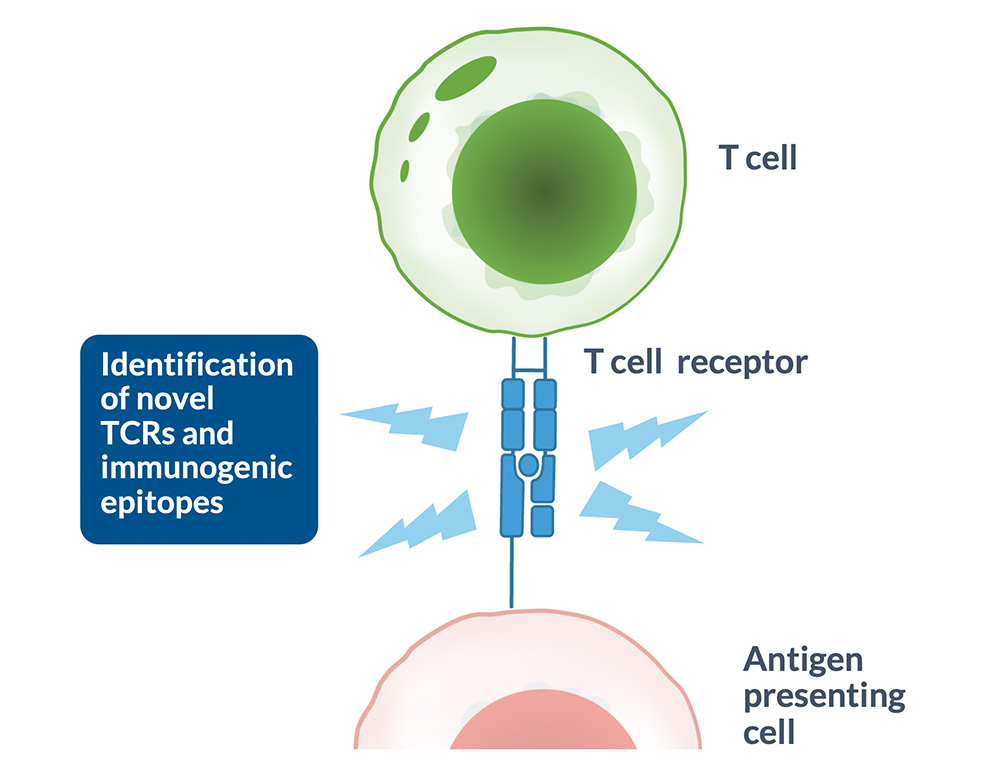
Costimulatory Coreceptor
- Improves T cell fitness and persistence by amplifying T cell activation signals
- Engages broader immune system and enables cytotoxic potential of CD4 cells
- Novel single chain design adds activating and pro-survival signaling to boost T cell functionality and persistence
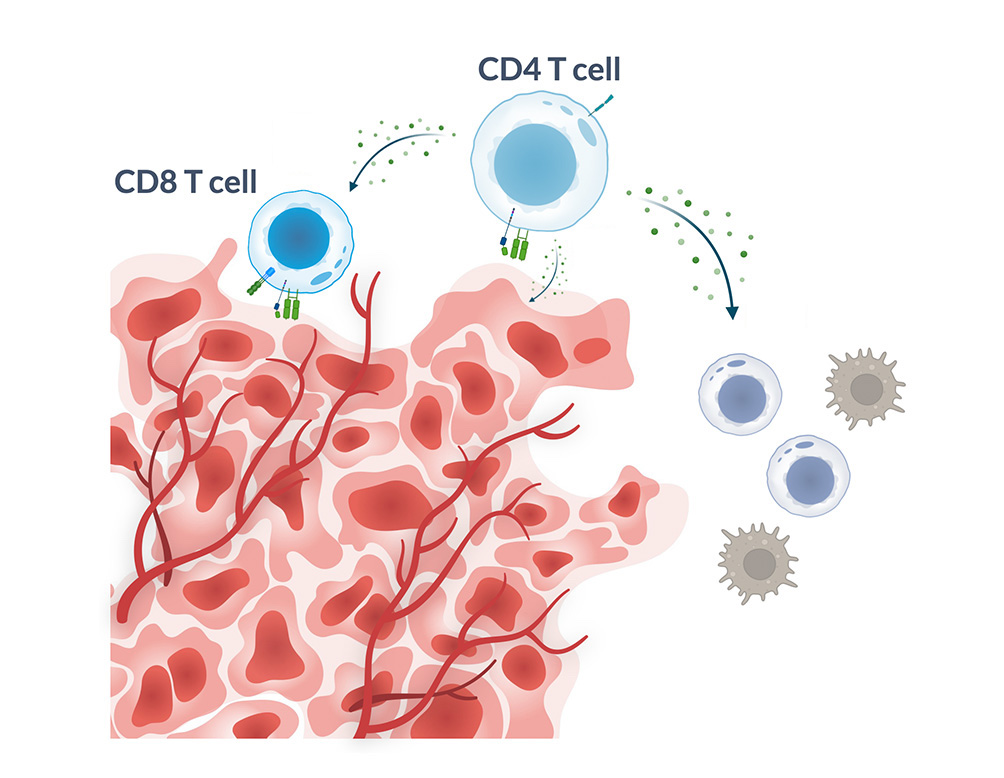
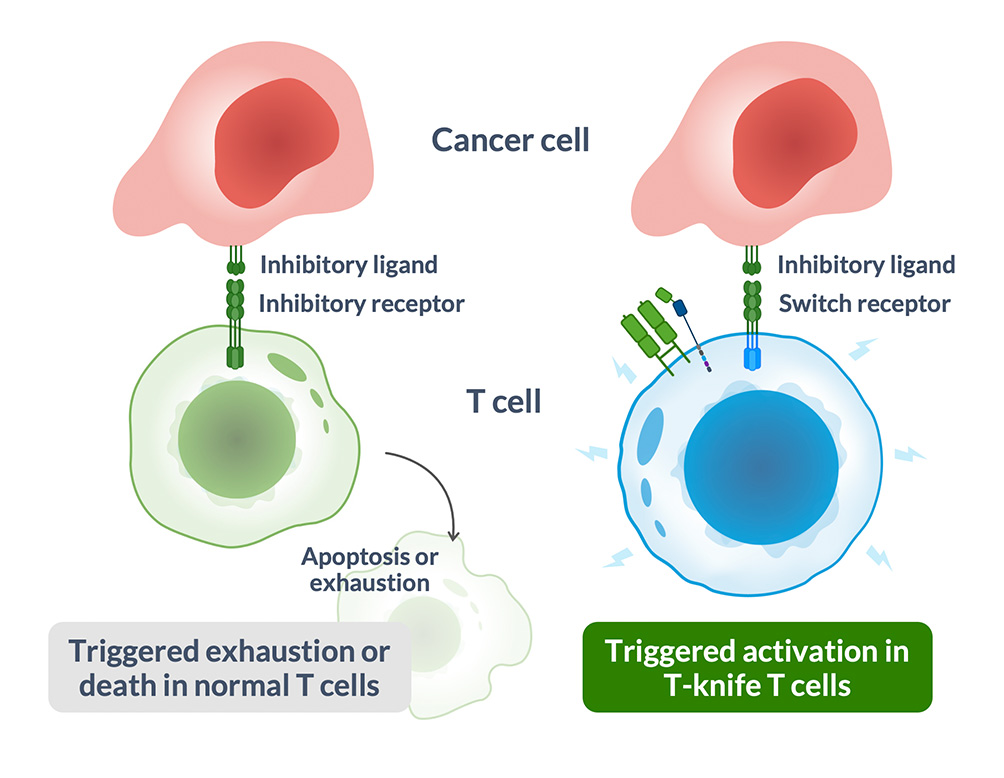
Tumor Microenvironment (TME) Armoring
- Novel switch receptors armor T cell to promote survival in TME
- Block inhibitory signals in tumors that kill T cells
- Trigger activating signals to prevent exhaustion, increase persistence
- Proprietary switch receptors are readily customizable for indication-specific targeting
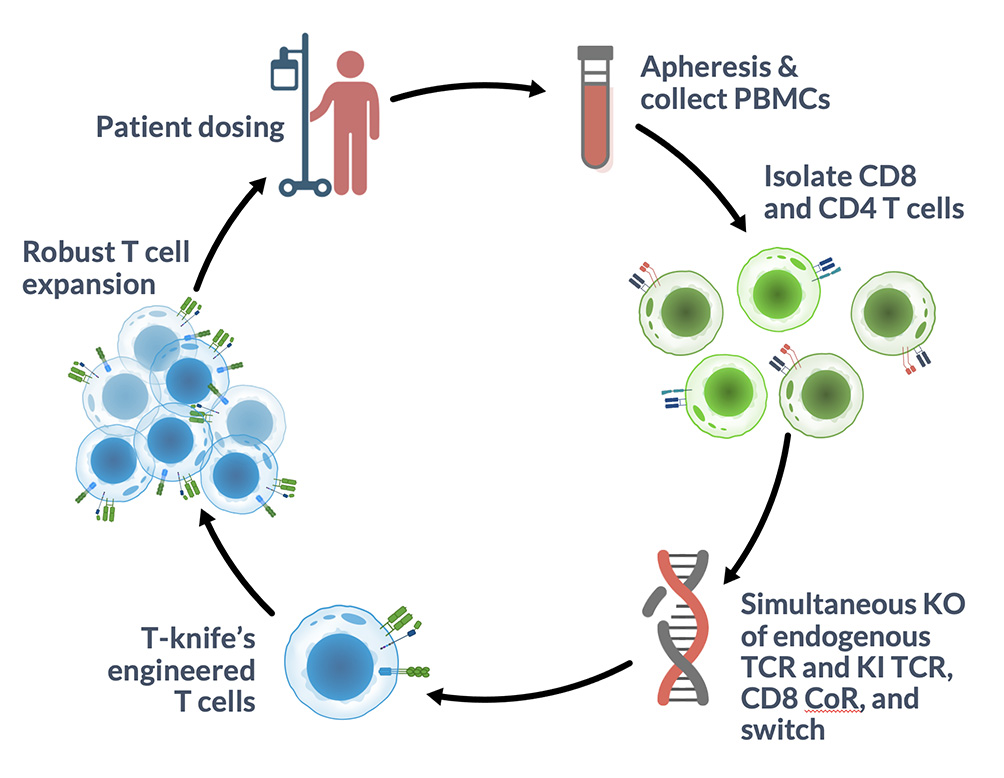
Non-Viral Manufacturing
- Improve cell yield and retain early memory phenotype, which is linked to better patient responses
- Endogenous TCR KO drives improved transgenic TCR expression
- Faster timelines from idea to patient with non-viral gene delivery methodologies